Small molecule SWELL1 complex induction improves glycemic control and nonalcoholic fatty liver disease in murine Type 2 diabetes | Nature Communications

An aqueous solution contains 30% w/v of urea , density of solution is 1.2 g/ml. Calculate mass of water in 100ml solution.

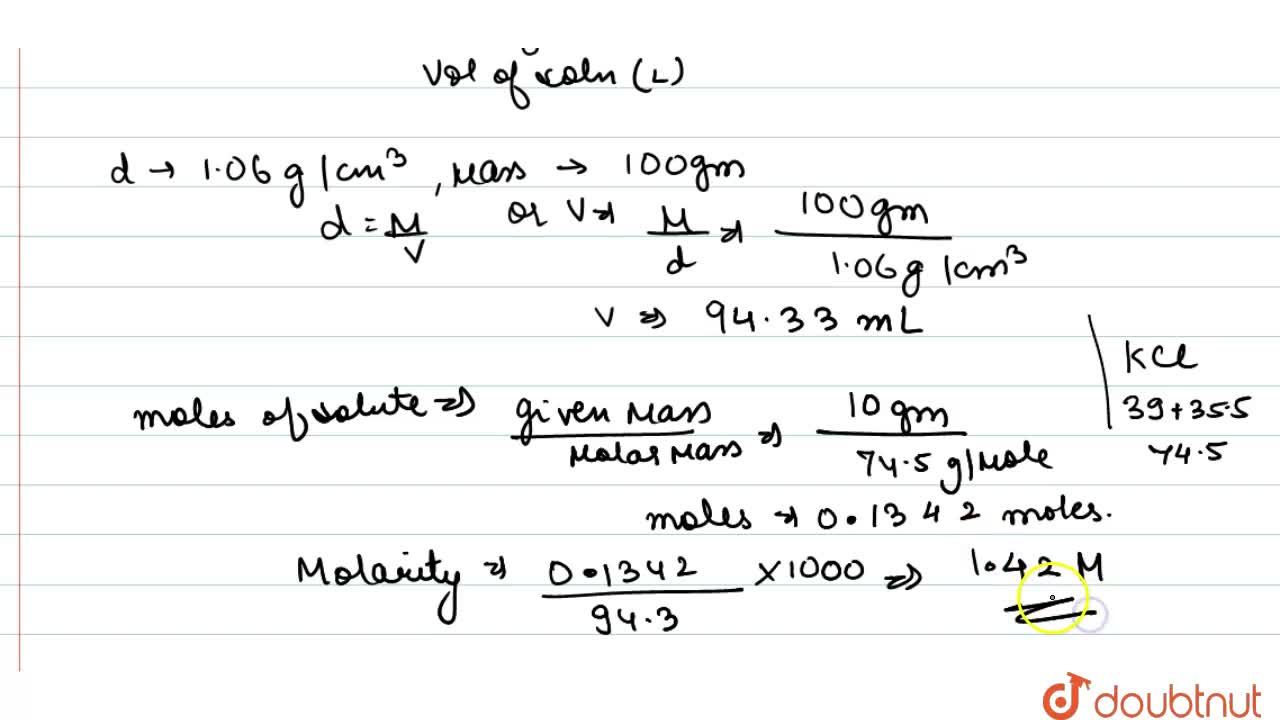
The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.

A solution of `KCl` has a density of `1.69 g mL^(-1)` and is 67% by weight. Find the denisty of ... - YouTube
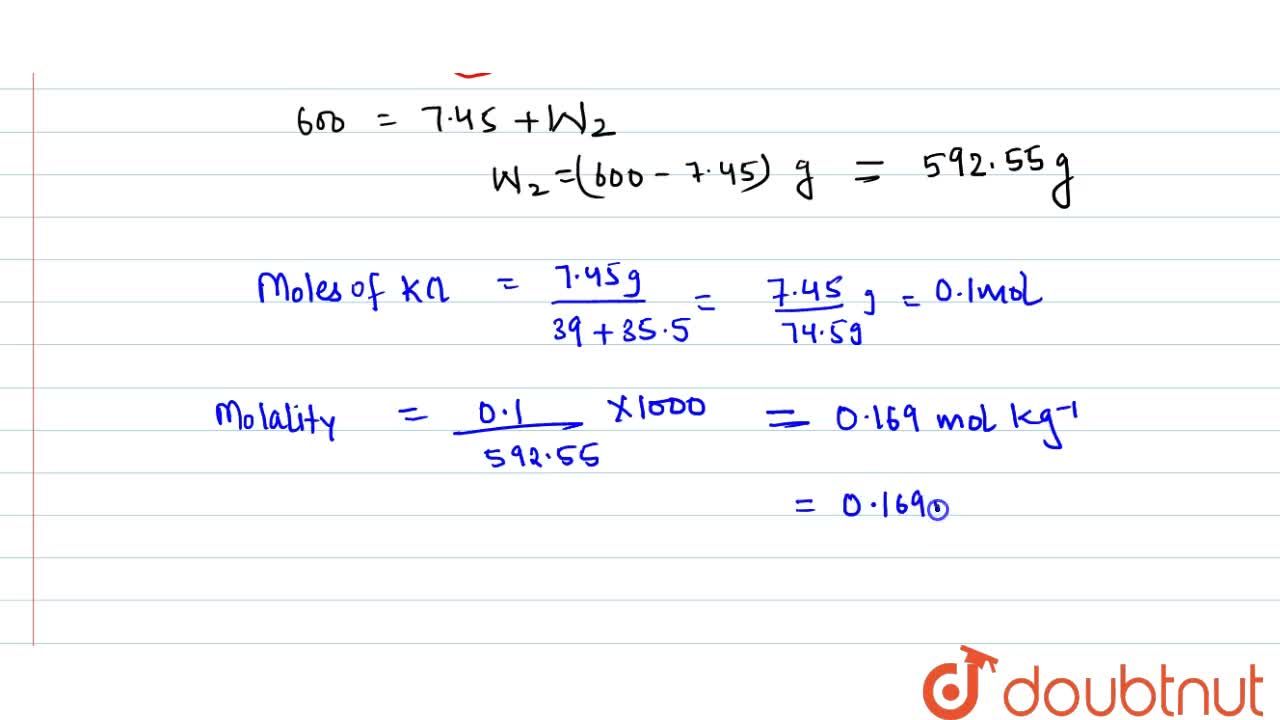
Calculate the molarity of KCl solution prepared by dissolving 7.45 g of KCl in 500 mL of the solution. (d(sol) = 1.2 g mL^(-1))

A solution of KCl has a density of 1.69 g mL^(-1) and is 67% by weight. Find the denisty of the solution if it is diluted so that the percentage by weight
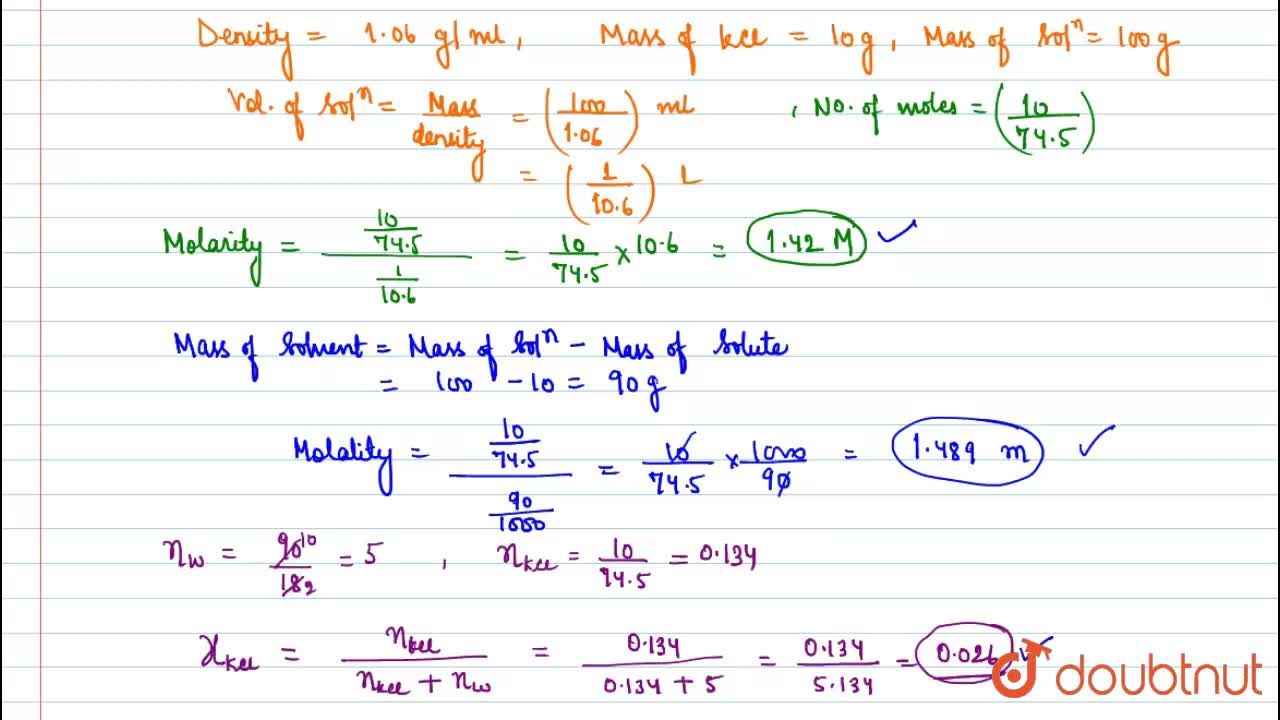
The density of a 10.0% by mass of KCl solution in water is 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in the solution.

The density of 3 M solution of NACl is `1.25 g mL^(-1)`. Calculate the molality of the solution. - YouTube
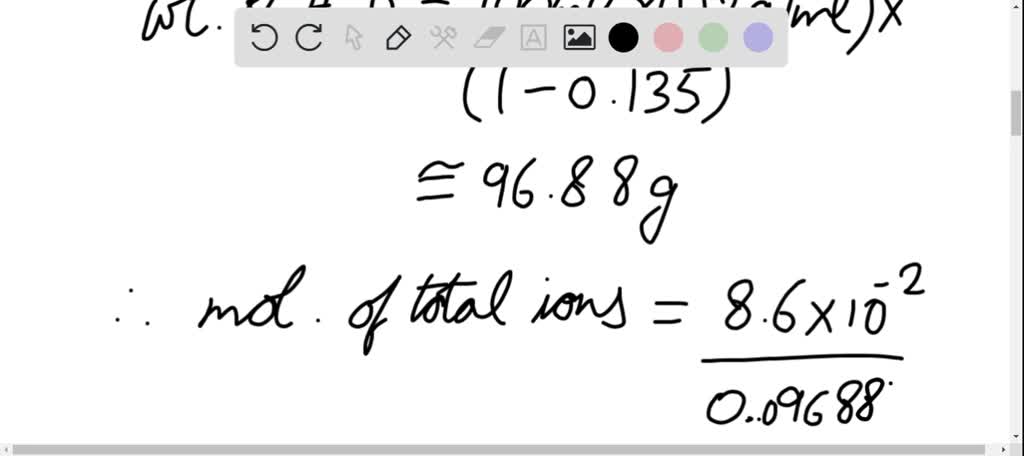
SOLVED:A 100.0 -mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g / mL . What would you add (solute or solvent) and what mass

Quantification of Zeta-Potential and Electrokinetic Surface Charge Density for Colloidal Silica Nanoparticles Dependent on Type and Concentration of the Counterion: Probing the Outer Helmholtz Plane | The Journal of Physical Chemistry C

The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.
Molecules | Free Full-Text | Galectin-1 Used in Assisted Reproduction—Embryo Safety and Toxicology Studies

Calculate the molality of the KOH solution having density 1.5 g/mL, when the molarity of the same - Brainly.in

Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors | Molecular Psychiatry

A solution is prepared by dissolving 4 g of NaOH to give 500 ml of it. Calculate the molality of the solution.

Aq KCL #solution of #density 1.2 g/ml has a #molality of 3.30 mol/kg. find #molarity. #jeemains2021 - YouTube