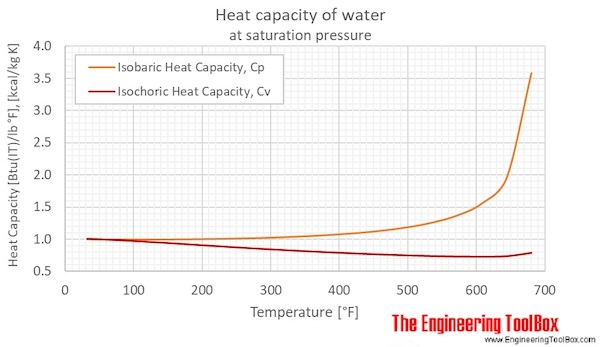
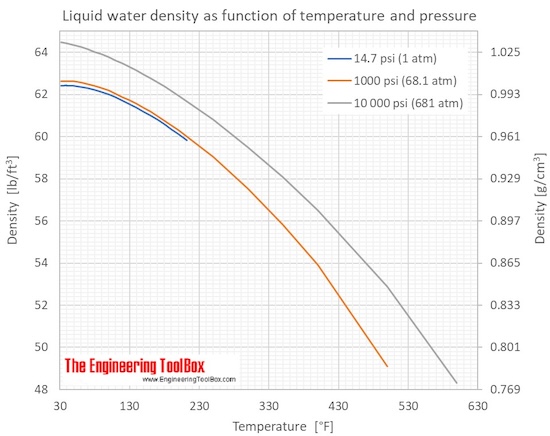
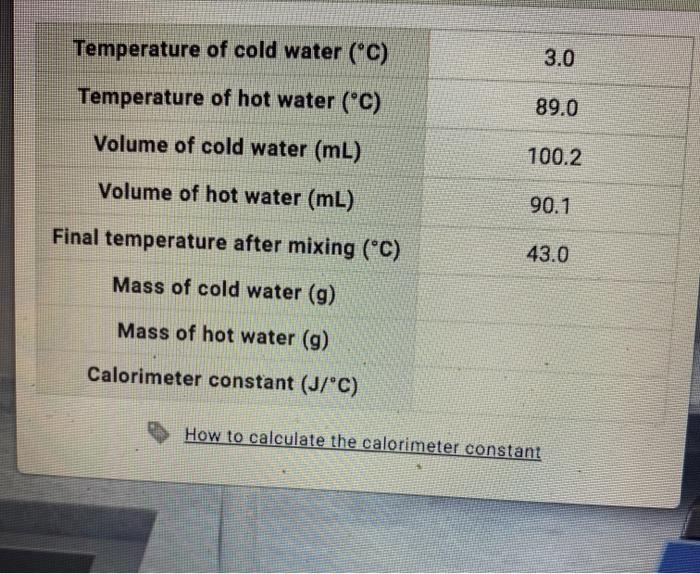
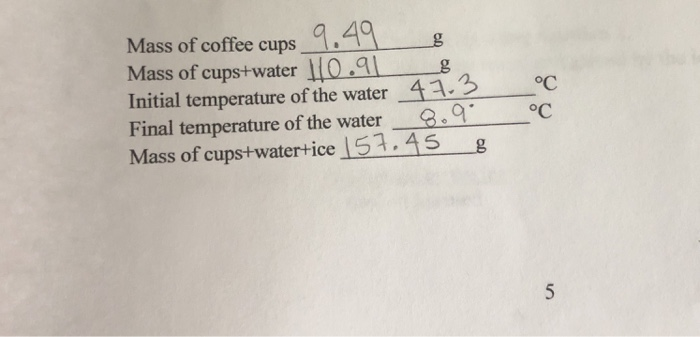Calculate the heat of fusion of ice from the following data of ice at `0^C` added to water. Mass... - YouTube
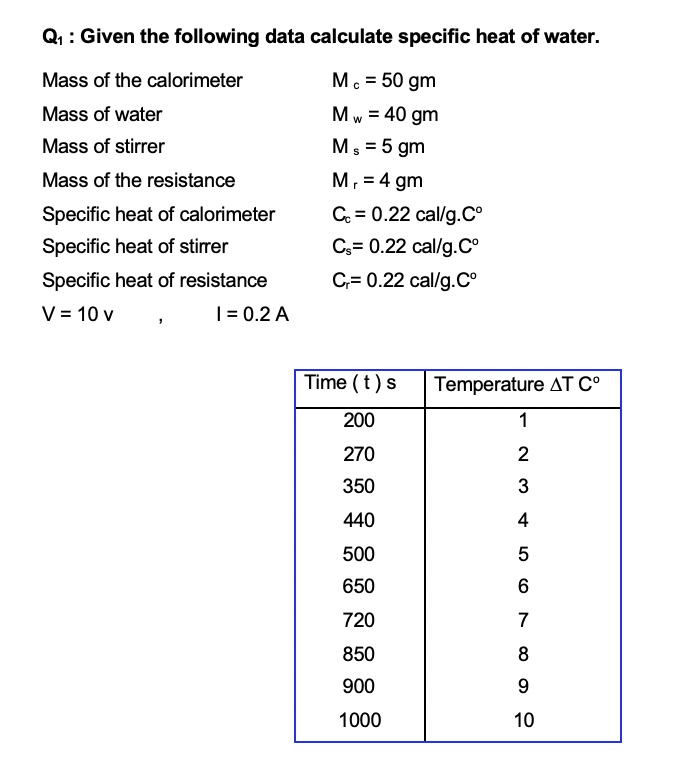
SOLVED: Q1 Given the following data calculate specific heat of water: Mass of the calorimeter Mass of water Mass of stirrer Mass of the resistance Specific heat of calorimeter Specific heat of
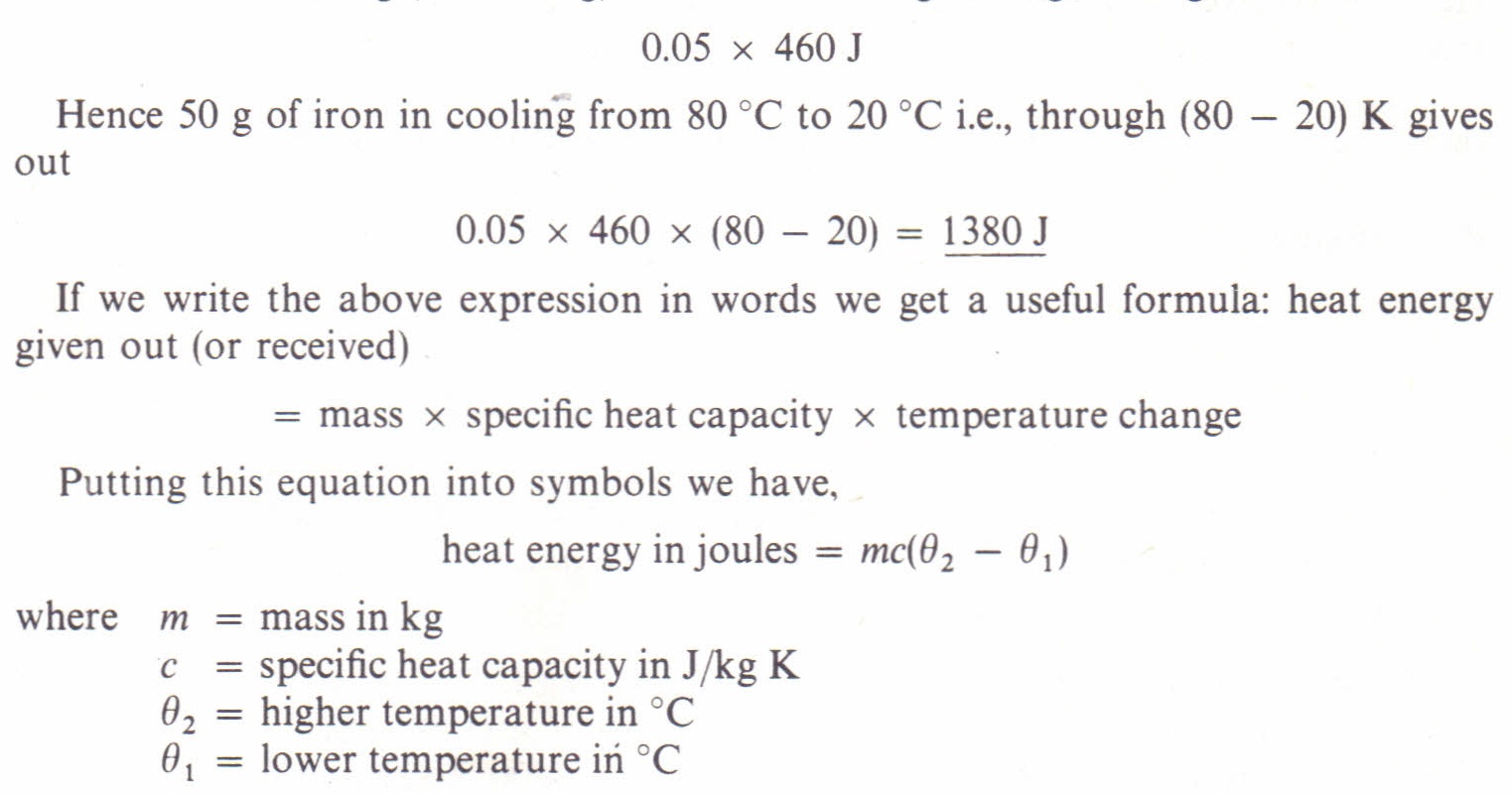
Cheat calculations Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

40 g of ice at 0^oC is used to bring down the temperature of certain mass of water at 60^oC to 10^oC . Find the mass of water used.[Specific heat capacity of

Question Video: Calculating the Heat Energy Transferred to Water Using Its Specific Heat Capacity | Nagwa

Hydrates & Anhydrates Overview, Formula & Examples | What Is an Anhydrate? - Video & Lesson Transcript | Study.com

Question Video: Determining the Mass of Water Lost When a Hydrated Compound of Cobalt(II) Sulfate Is Heated | Nagwa
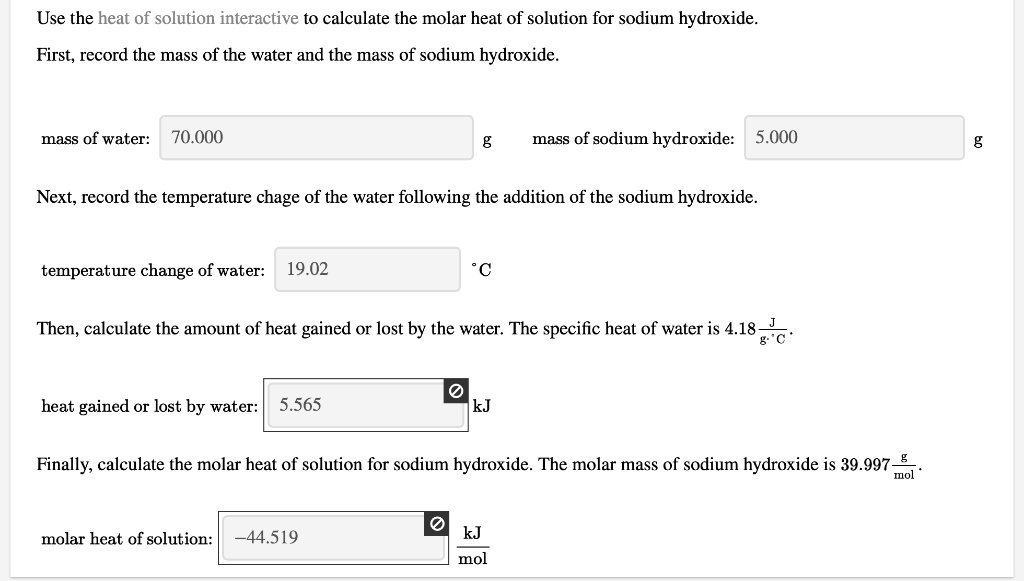
SOLVED: Use the heat of solution interactive to calculate the molar heat of solution for sodium hydroxide. First, record the mass of the water and the mass of sodium hydroxide: mass of
A 2kg piece of iron at 20°C is provided with 30Kcal of heat and is immediately dropped in 1 liter of water at 20°C. What is the final temperature? - Quora