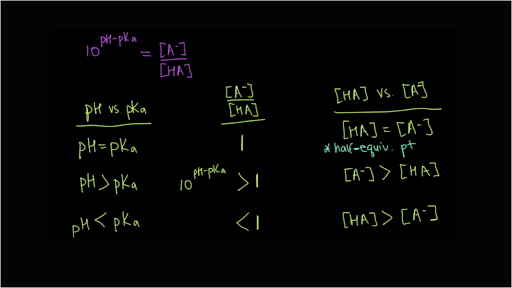
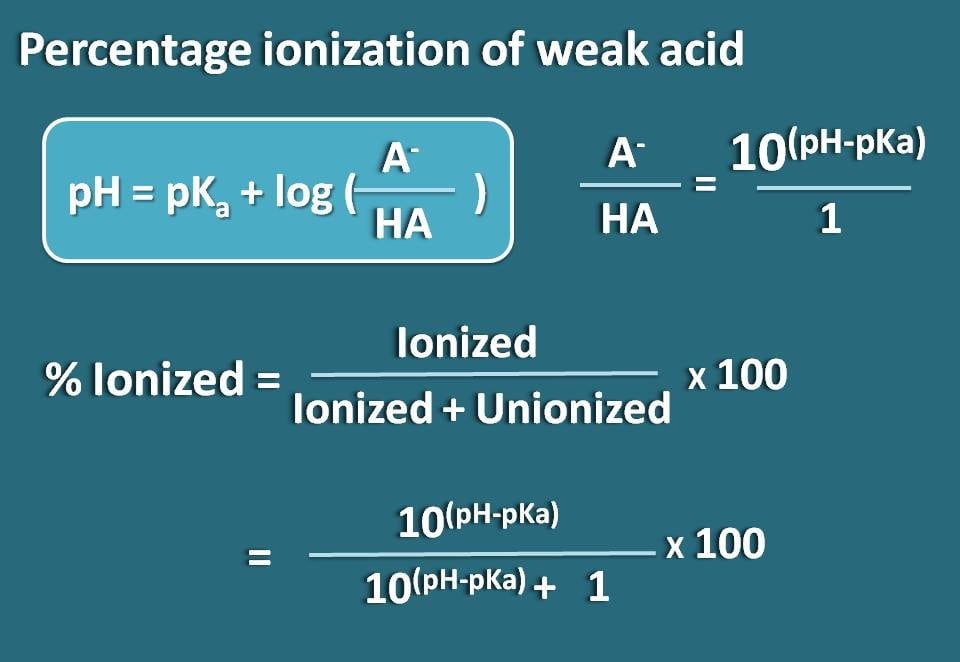
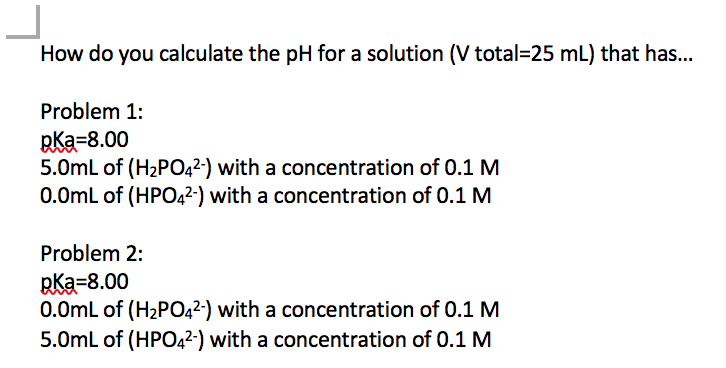
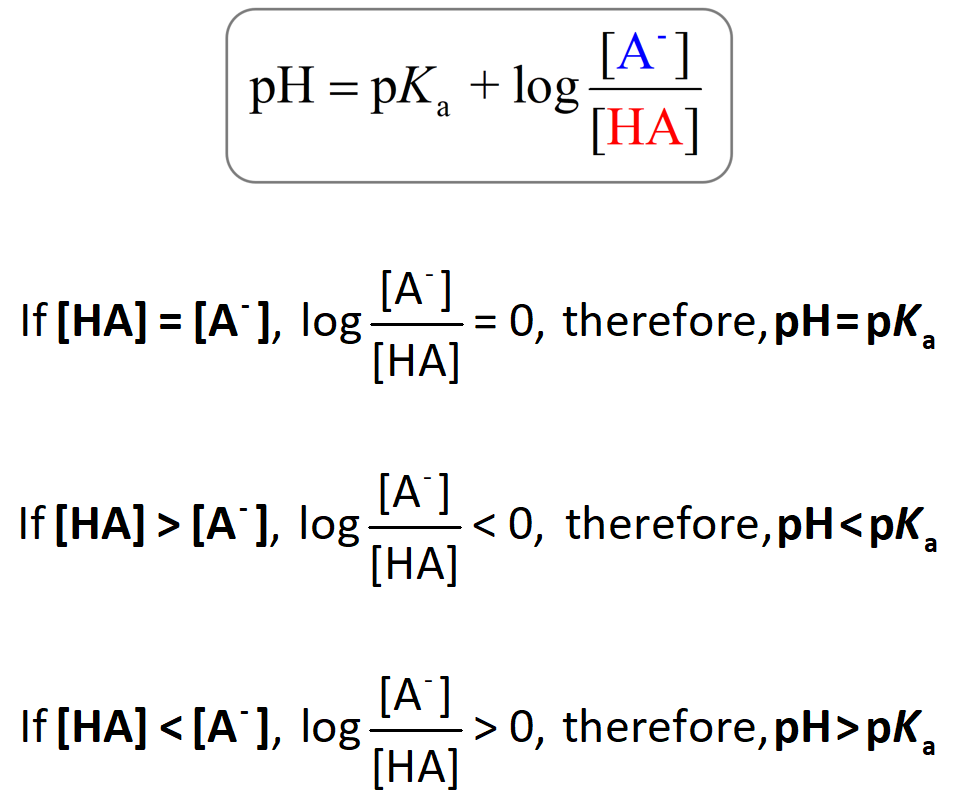
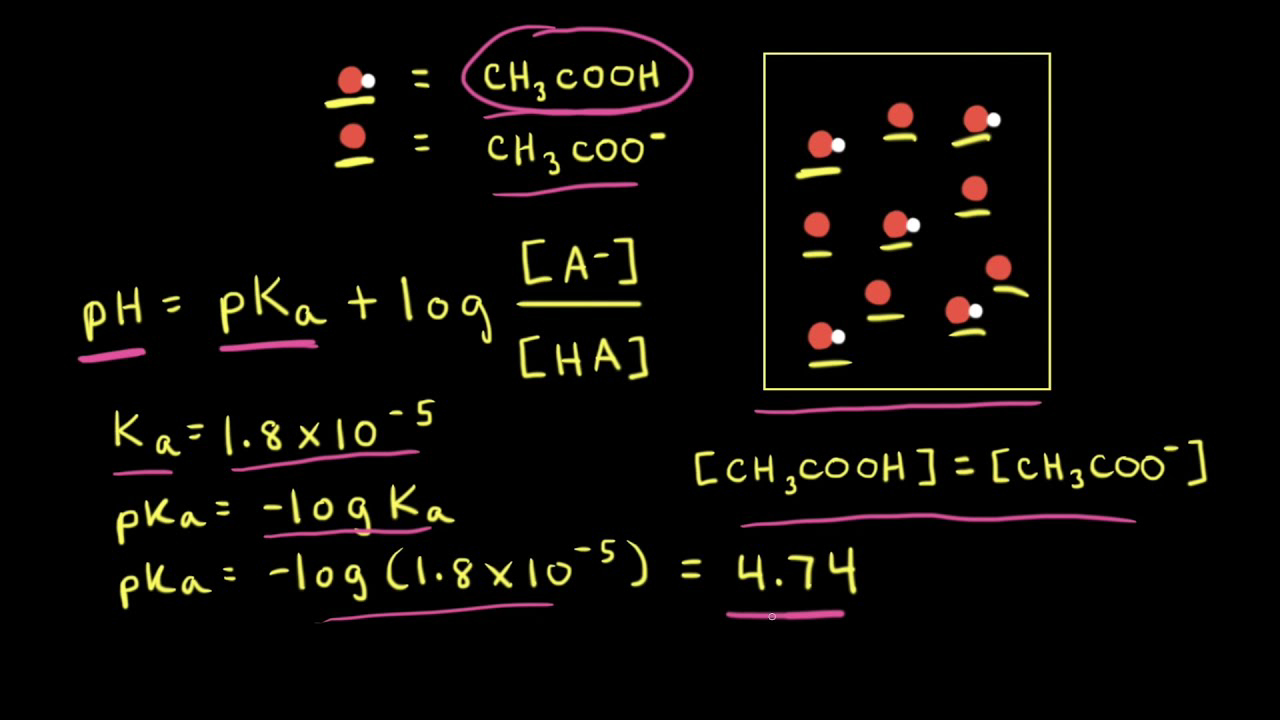
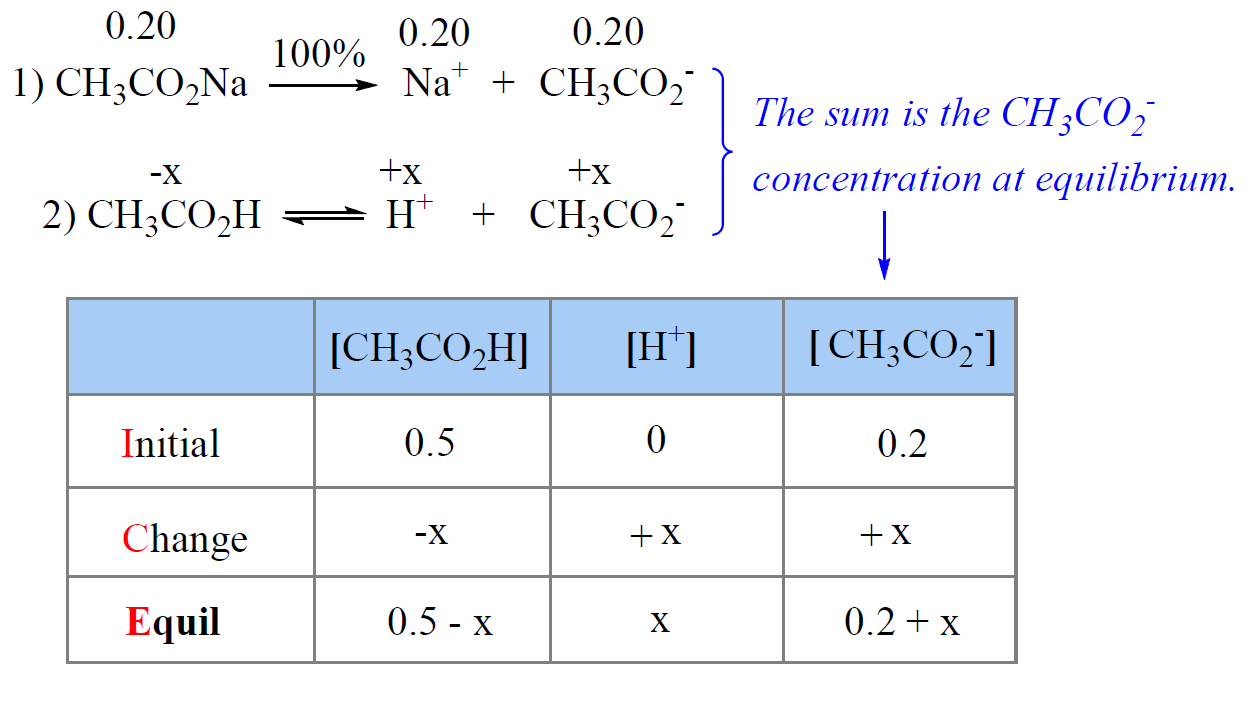
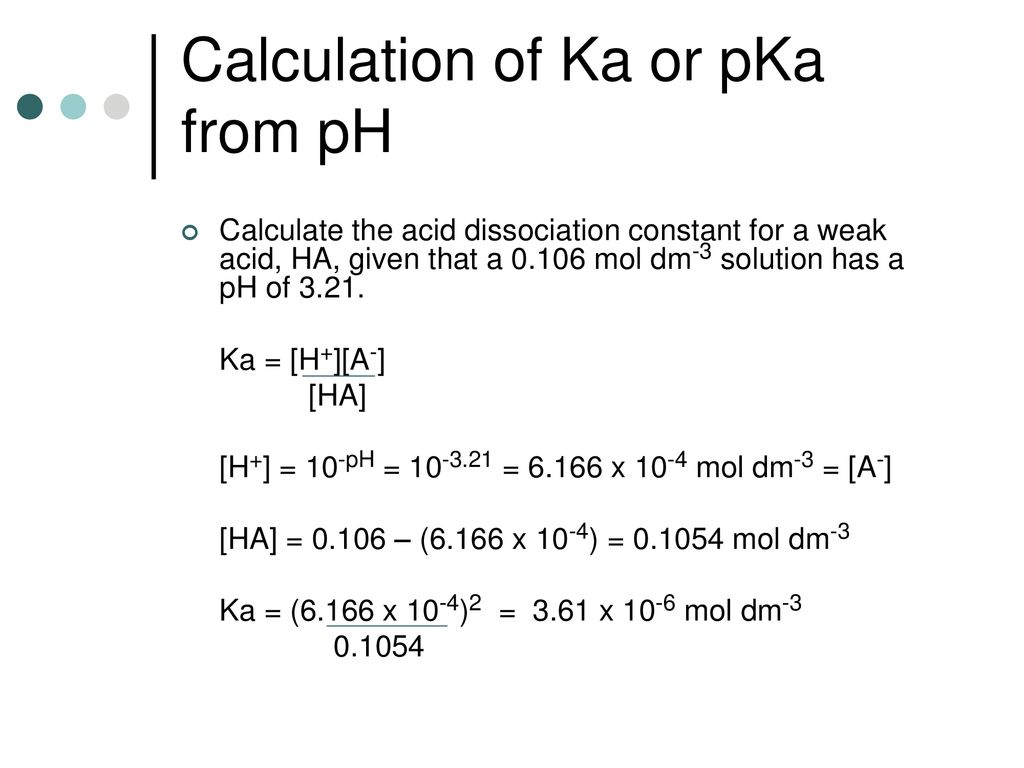
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
![SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice](https://cdn.numerade.com/ask_previews/f6745ba3-6b77-4e7a-9de2-7d980958d194_large.jpg)
SOLVED: Calculate Ka and pKa of the acid using pH and molarity.moles unknown acid = 0.001215 molar mass of acid = 172.84molarity = 0.243 mol/LpH= 2.06kA= [A-][H3O+] / [HA]please include a rice

Problem #1 - What is the pH of each of the following solutions? a) M HCl Strong acids completely dissociate in solution therefore the total concentration. - ppt download

pKa & pH Values| Functional Groups, Acidity & Base Structures - Video & Lesson Transcript | Study.com


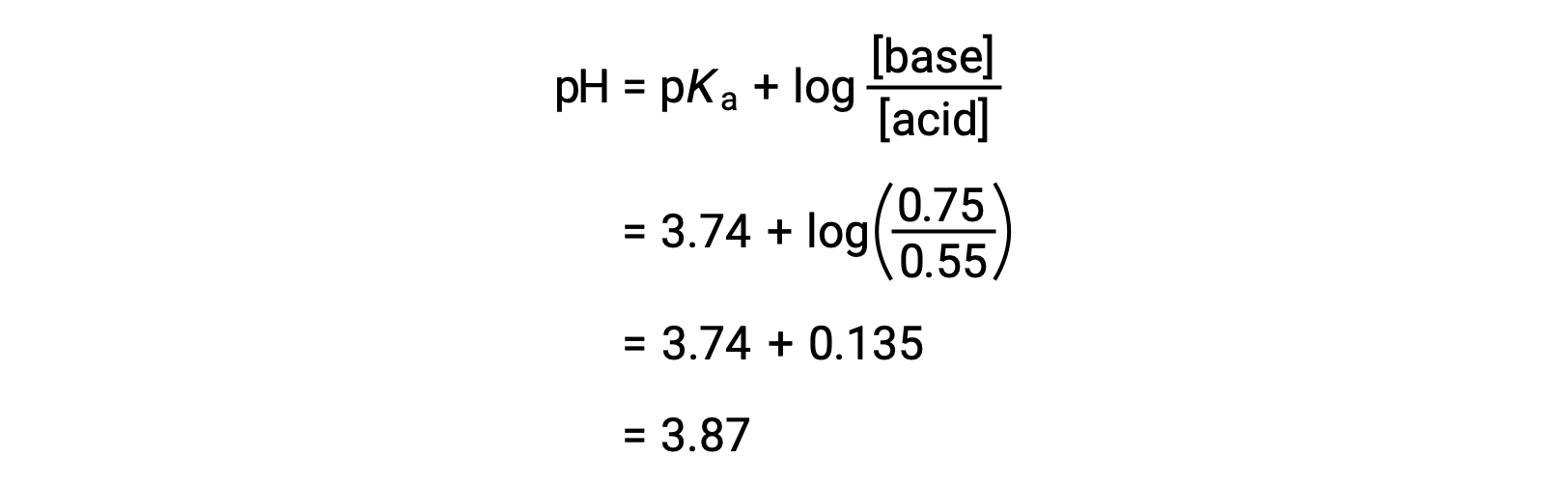
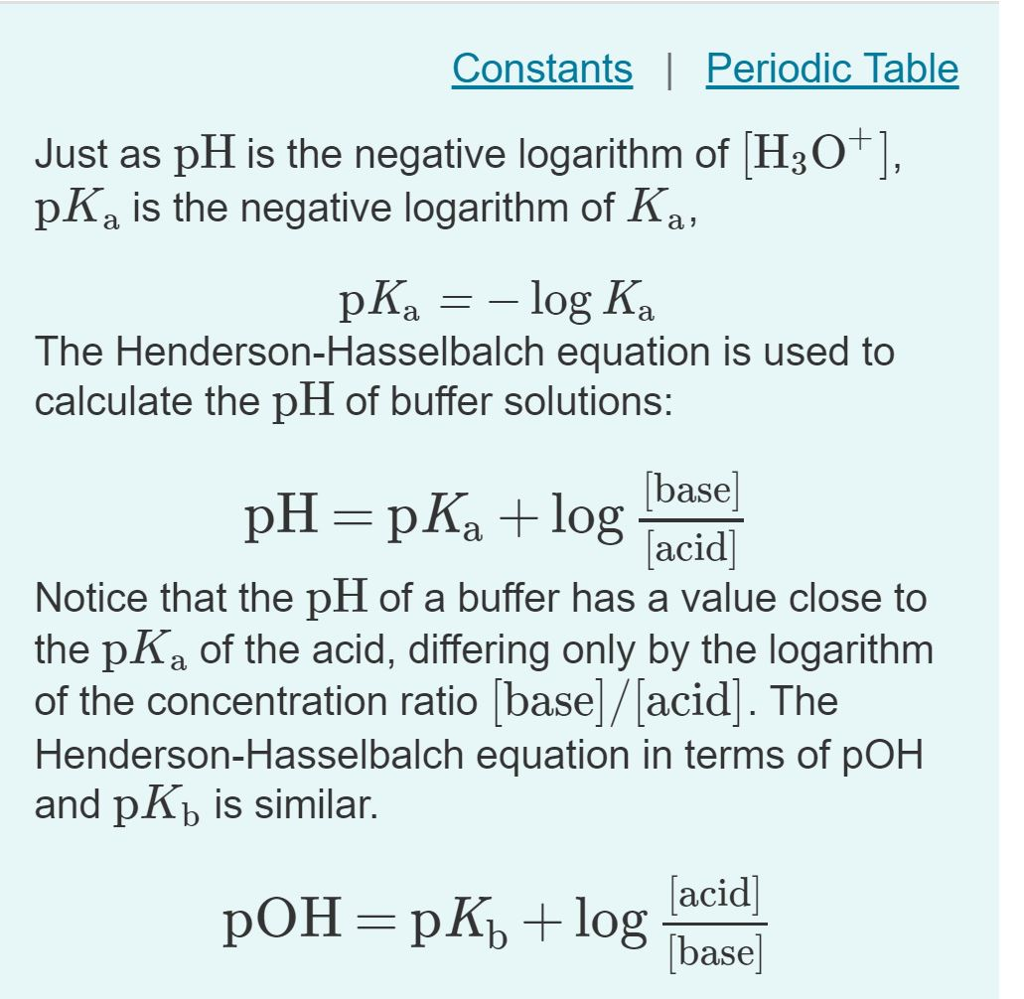
![Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com](https://media.cheggcdn.com/media/375/3755951c-9b2e-4931-a914-33bb100113d8/php72x3Cn.png)