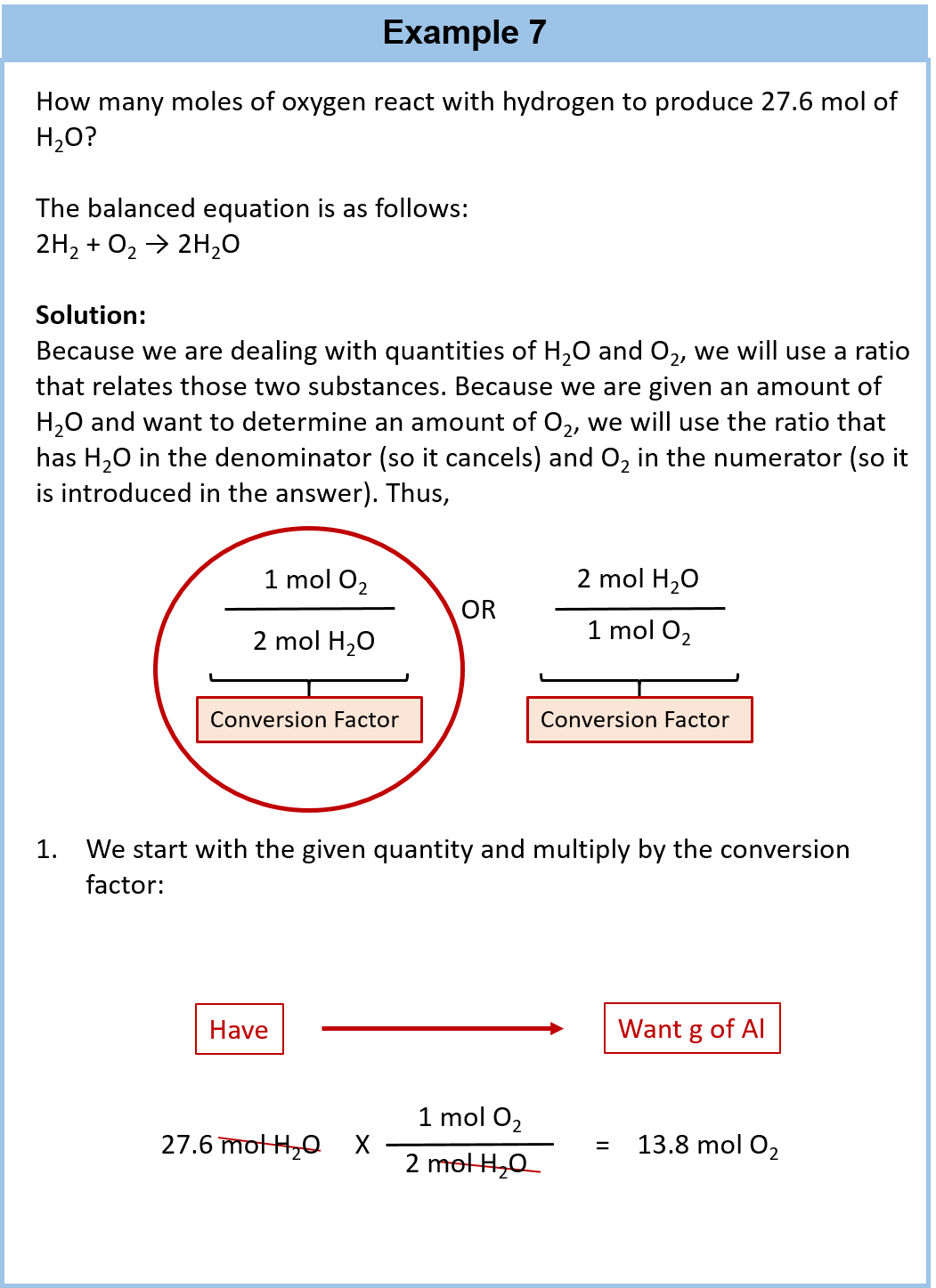
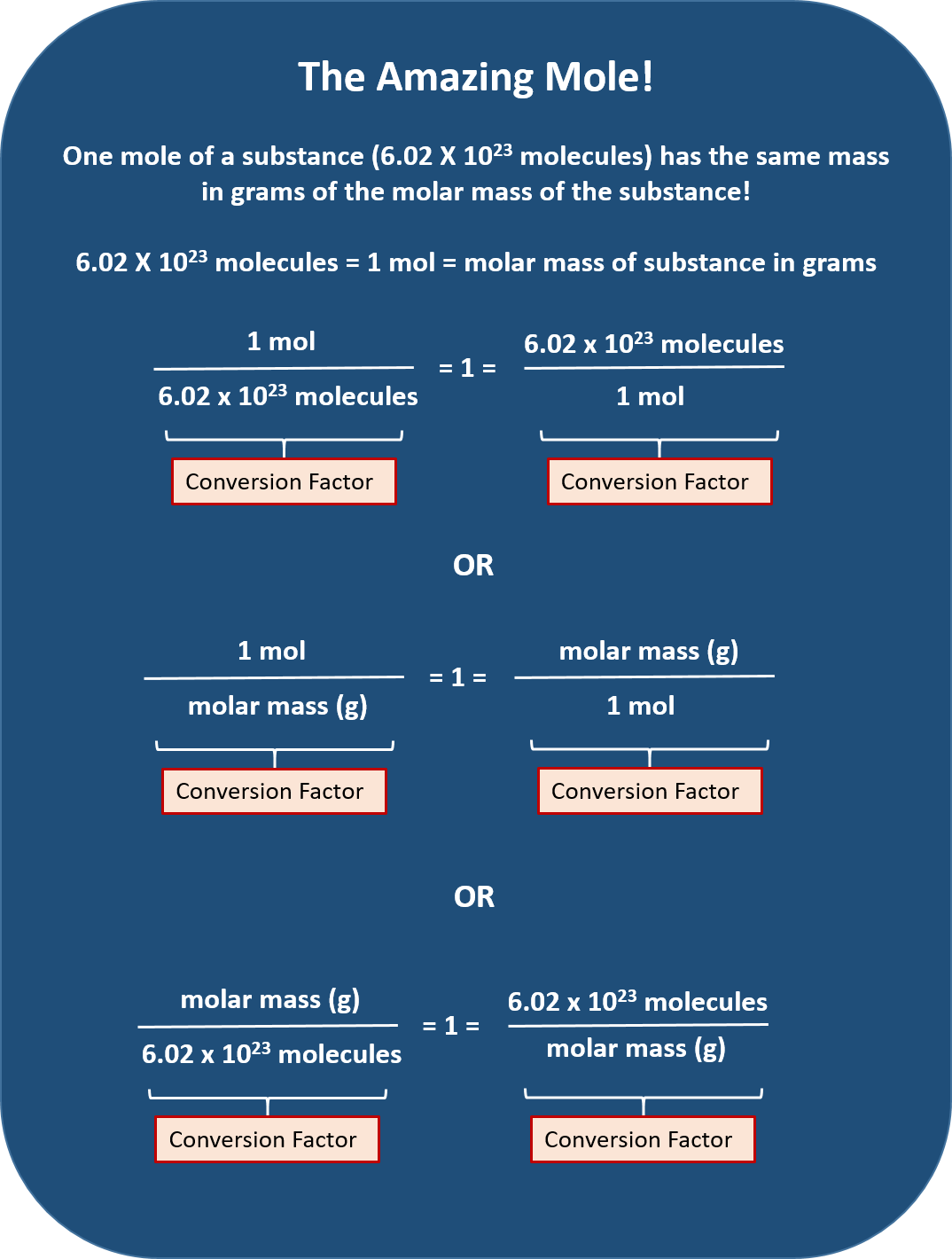
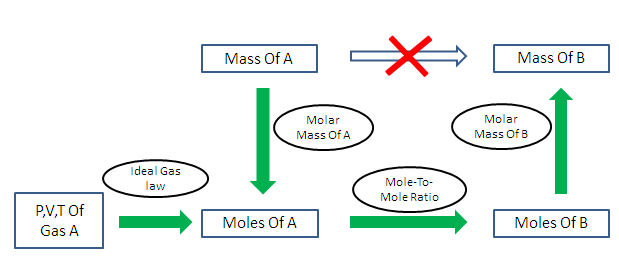
Analysis of Two Definitions of the Mole That Are in Simultaneous Use, and Their Surprising Consequences | Journal of Chemical Education
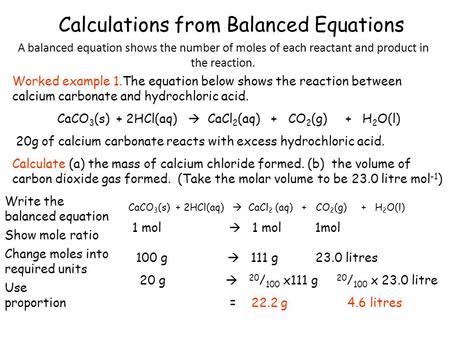
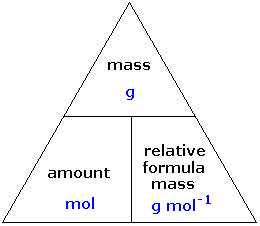
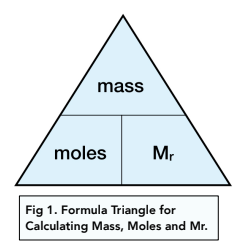
STANDARD GRADE CHEMISTRY CALCULATIONS Calculations involving the mole. 1 mole of a solid substance is the formula mass of the substance in grams. This. - ppt download
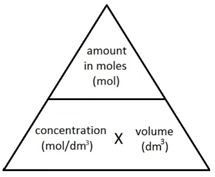
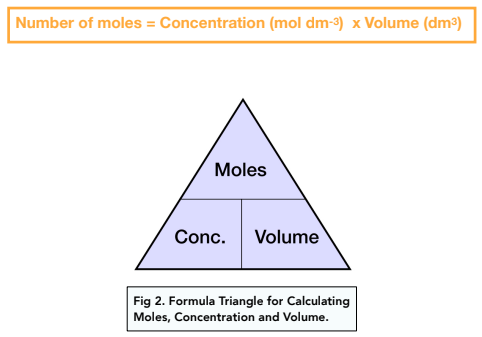
1:34 (Triple only) understand how to carry out calculations involving amount of substance, volume and concentration (in mol/dm³) of solution - TutorMyself Chemistry