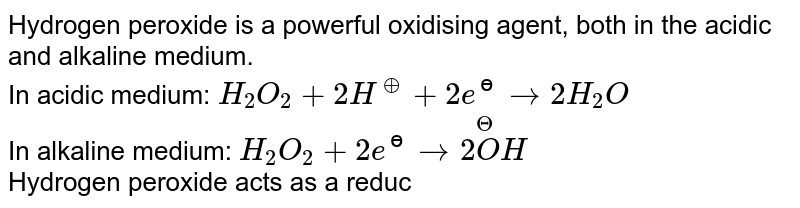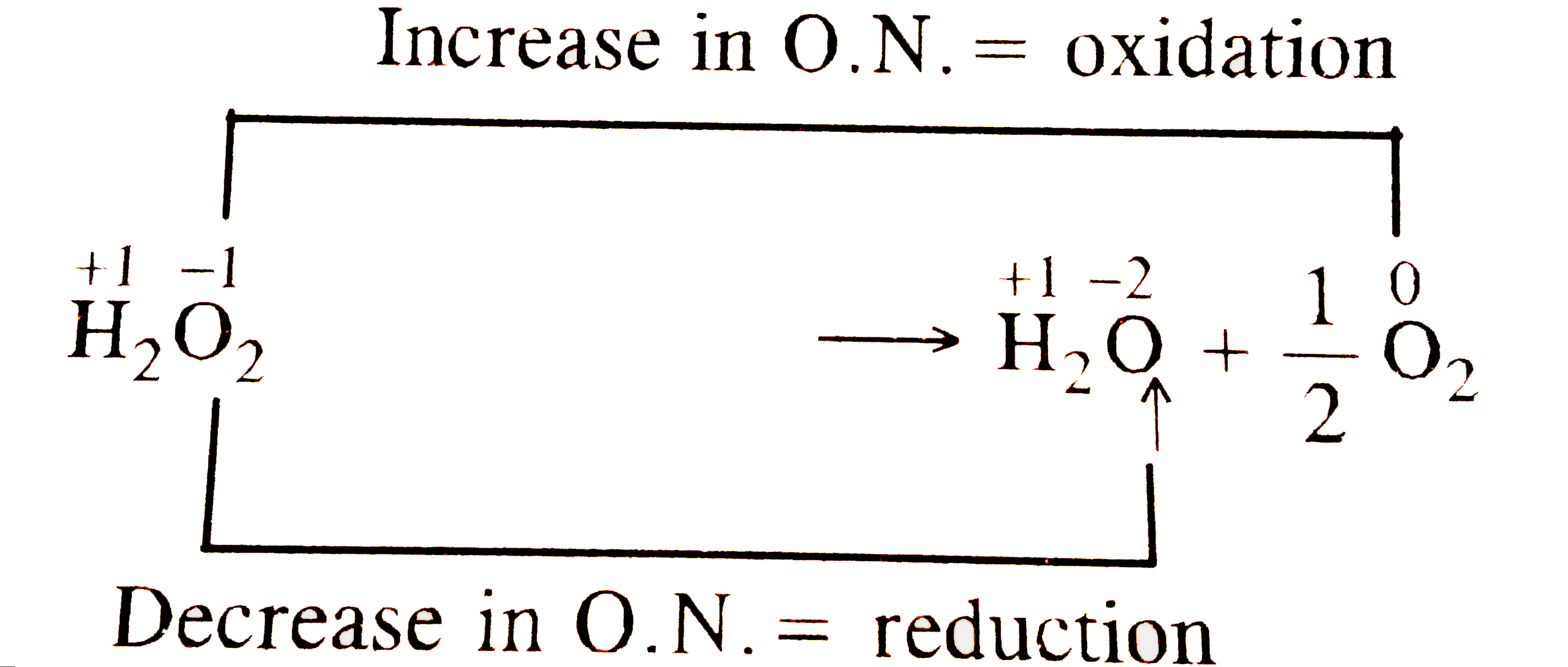Relate chemical activity to oxidizing and reducing strength. Explain the concept of disproportionation. - ppt download
An acidic solution of hydrogen peroxide behaves as an oxidising as well as reducing agent. Illustrate it with the help of a chemical equation. - Sarthaks eConnect | Largest Online Education Community

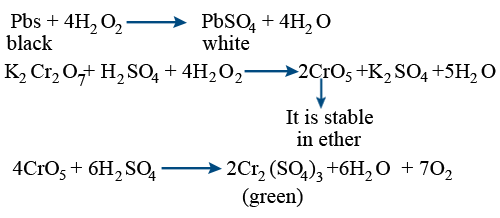
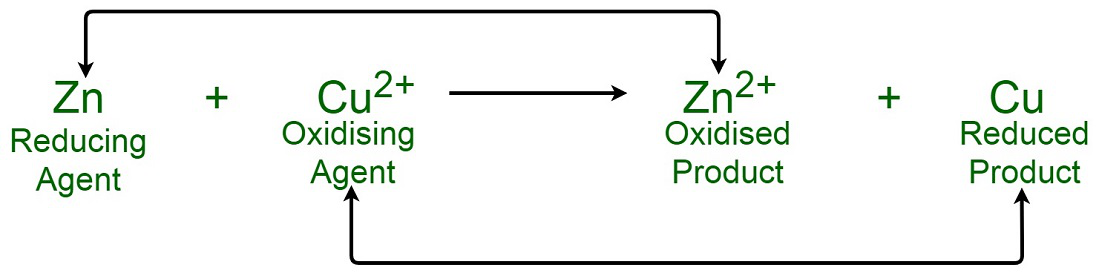
Hydrogen peroxide acts both as an oxidising and as a reducing agent depending upon the nature of the reacting species. In which of the following cases H(2)O(2) acts as a reducing agent
Hydrogen peroxide acts as both a reducing agent and oxidizing agent depending upon the nature of the reacting species. In which case does peroxide act as a reducing agent in acid medium? -
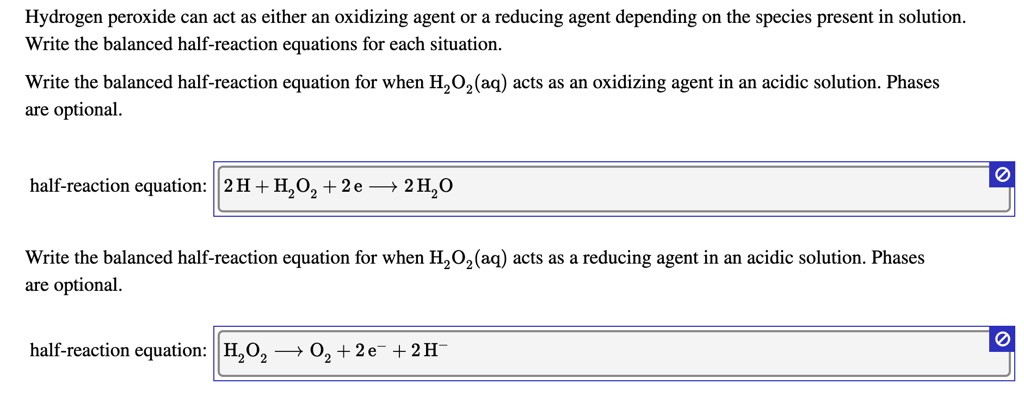
SOLVED: Hydrogen peroxide can act as either an oxidizing agent Or a reducing agent depending on the species present in solution Write the balanced half-reaction equations for each situation Write the balanced
While sulphur dioxide and hydrogen peroxide can actas oxidising as well as reducing agents in theirreactions, ozone and nitric acid act only as oxidants.This is because(a) in SO2 and H2O2, S and